- Un estudio liderado por el Dr. Miguel Sánchez del IIBM descubre el mecanismo que permite la coordinación entre el retículo endoplásmico y el citoesqueleto, esencial para la diferenciación y el correcto funcionamiento de las células
- El hallazgo, publicado en la revista Cell Reports, ofrece una nueva perspectiva sobre los procesos celulares y moleculares implicados en diversas enfermedades, incluidos síndromes neurodegenerativos, y podría contribuir al desarrollo de estrategias terapéuticas más efectivas
El interior de las células está organizado en diferentes compartimentos, conocidos como “orgánulos”, que desempeñan funciones específicas. Uno de estos orgánulos es el retículo endoplásmico (RE), una compleja red de membranas que realiza tareas esenciales, como la formación y maduración de al menos el 30% de las proteínas de la célula. Esto incluye especialmente las proteínas que se encuentran en la superficie celular y aquellas que se secretan al exterior. La arquitectura de esta red (la figura que acompaña el texto) debe adaptarse a la forma de la célula, que puede variar desde pequeños linfocitos esféricos hasta neuronas con prolongaciones que miden varios centímetros. Además, el RE debe ajustarse a las necesidades cambiantes de la célula. Así, cuando el RE no funciona de forma óptima, una situación conocida como “estrés del RE”, las células tienden a reestructurarlo y aumentar su volumen.
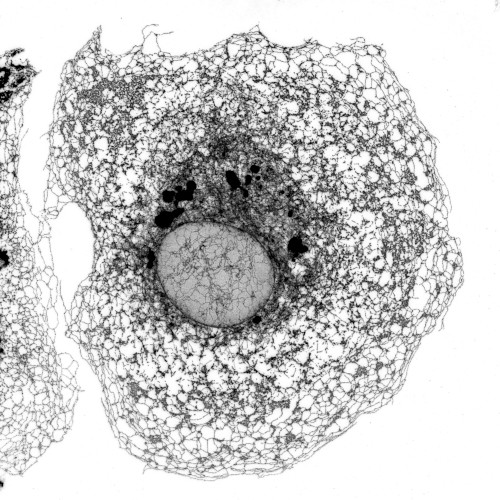
Microscopía confocal de alta resolución del retículo endoplásmico de una célula de riñón de mono verde.
Desde hace tiempo se sabe que el control preciso de la arquitectura de esta red de membranas es crucial para la supervivencia y el correcto funcionamiento de los diferentes tipos de células en el organismo. Sin embargo, aún se desconoce en gran medida cómo se lleva a cabo este control y cómo se coordina con los cambios en la forma de la célula.
Un reciente estudio liderado por el científico Miguel Sánchez del Instituto de Investigaciones Biomédicas Sols-Morreale (IIBM), centro mixto del Consejo Superior de Investigaciones Científicas (CSIC) y la Universidad Autónoma de Madrid (UAM), y publicado en la revista Cell Reports, describe un mecanismo clave para el control coordinado de la arquitectura celular. Los investigadores utilizaron una metodología avanzada de microscopía de alto contenido (high-content microscopy), que permite capturar imágenes del RE en cientos de miles de células y analizar su estructura de manera automatizada. Esto facilita la realización, en tiempos razonables, de un cribado en el que se anula la expresión de miles de genes para estudiar su impacto en la función celular.
El estudio reveló que la proteína PERK desempeña un papel más amplio de lo que se pensaba anteriormente, ya que no solo limita la producción de nuevas proteínas cuando se activa por estrés del RE, sino que también es esencial para que la expansión del RE se acomode correctamente dentro del volumen celular. “Uno de los primeros hallazgos inesperados de este estudio fue que cuando PERK se activa, no sólo reduce la síntesis de proteínas, sino que también relaja el anclaje entre el RE y los microtúbulos del citoesqueleto, permitiendo que el RE se expanda y se distribuya de manera adecuada dentro de la célula”, explica Miguel Sánchez. Los microtúbulos implicados en el proceso de unión con el RE son los denominados no centrosomales, que están relacionados con funciones esenciales de la célula como la orientación y la polarización celular.
Los investigadores se preguntaron si la activación de PERK también influiría en la estabilidad de los propios microtúbulos. “Aquí llegó otro hallazgo inesperado”, apunta el director del estudio. “Descubrimos que el grado de anclaje entre el RE y los microtúbulos, que está fuertemente influenciado por la actividad de PERK, también afecta la estabilidad de estos microtúbulos”. En células sometidas a estrés del RE —por ejemplo, tras la exposición a tunicamicina, una sustancia que provoca la acumulación de proteínas inmaduras en el RE—, PERK desactiva la maquinaria de síntesis de proteínas y debilita la unión del RE con los microtúbulos, facilitando su expansión física. Pero debilitar el anclaje RE-MTs tiene un efecto adicional: disminuye la estabilidad de los propios microtúbulos no centrosomales, en un proceso de retroalimentación negativa. Esto confirma que el control de la arquitectura del RE y la estabilidad y dinámica de los microtúbulos están estrechamente coordinados y muy relevante, que podemos manipular artificialmente este sistema”, detalla el investigador.
Una nueva pieza en el puzle de múltiples enfermedades
Este sistema dinámico explica otra observación notable del estudio. “La morfología y el comportamiento móvil de la célula están, en parte, condicionados por el estado funcional del RE y el control de la síntesis de proteínas a través de PERK”, explica Miguel Sánchez. “Cuando las células experimentan un alto estrés del RE y activan PERK, reducen el anclaje del RE a los microtúbulos no centrosomales para permitir su expansión. Sin embargo, esto también disminuye la estabilidad y abundancia de estos MTs, lo que hace que las células adopten un estado más estático, sin una organización claramente polarizada y con menor capacidad de migración”, añade. “Por el contrario, las células con una actividad reducida de PERK mantienen un alto grado de anclaje entre el RE y los MTs. Esto puede dificultar la reorganización del RE, pero también estabiliza los MTs no centrosomales, promoviendo una morfología con protrusiones grandes y estables”.
Estos hallazgos son muy relevantes dada la importancia de estos mecanismos en la fisiología de las células, lo que podría tener implicaciones importantes en enfermedades neurológicas como la paraparesia espástica o ciertos síndromes de demencia. Asimismo, los autores han observado que la reducción de la actividad de PERK estabiliza la formación de los axones durante la diferenciación neuronal, lo que podría influir en procesos como la memoria y el aprendizaje. También podrían ayudar a comprender mejor el papel del estrés del RE en cáncer. “La capacidad de las células cancerosas para adaptarse a este desequilibrio no solo influye en su supervivencia, sino que también puede modificar su comportamiento migratorio, un factor clave en la metástasis”, explica Chris Bakal. “El nivel de detalle que estamos alcanzando en la comprensión de estos procesos —y, sobre todo, cómo se relacionan entre sí— podría allanar el camino para encontrar nuevas estrategias terapéuticas en distintas enfermedades”, concluye Miguel Sánchez.
Esta investigación ha contado con la colaboración de los laboratorios de Chris Bakal en el Institute of Cancer Research (ICR) en Londres, y de Miguel Ángel del Pozo y Jesús Vázquez en el Centro Nacional de Investigaciones Cardiovasculares (CNIC) en Madrid; y ha contado con financiación de: The Wellcome Trust, Cancer Research UK, la Asociación Española Contra el Cáncer, el Ministerio de Ciencia, Innovación y Universidades, y la Fundación La Caixa.
Dada la relevancia de este trabajo, el departamento de Comunicación del CSIC ha publicado esta nota de prensa en su página de Actualidad.
Imagen de la portada: Micrografías de superresolución de una célula epitelial de mama humana, mostrando la relación entre las arquitecturas del retículo endoplásmico (ER; verde) y el citoesqueleto de microtúbulos (MTs; magenta).
Miguel Sánchez-Álvarez, Chris Bakal, et al,. PERK-dependent reciprocal crosstalk between ER and non-centrosomal microtubules coordinates ER architecture and cell shape. Cell Reports, 2025. https:// doi.org/10.1016/j.celrep.2025.115590