- The group led by Dr. Bruno Sainz of the Instituto de Investigaciones Biomédicas Sols-Morreale publishes a study in the Journal of Nanobiotechnology that describes a new nanoemuslion that converts pro-tumor-associated macrophages into pro-inflammatory anti-tumor macrophages, decreasing pancreatic cancer tumor burden and metastasis
In a study published in the Journal of Nanobiotechnology, researchers led by Dr. Adrian Palencia-Campos (1st author) and Dr. Bruno Sainz Anding, head of the Cancer Stem Cells and Fibroinflammatory Microenvironment Group of the Instituto de Investigaciones Biomédicas Sols-Morreale (IIBM), CSIC-UAM, unveil a novel therapeutic approach for pancreatic ductal adenocarcinoma (PDAC), one of the deadliest cancers worldwide. The team demonstrated the power of lipid nanosystems to reprogram tumor-associated macrophages (TAMs), shifting them from tumor-promoting to tumor-fighting states. This innovative treatment not only reduced primary tumor growth but also significantly decreased liver metastases in preclinical models.
The nanosystems, composed of vitamin E and sphingomyelin, delivered the TGF-βR1 inhibitor Galunisertib directly to the PDAC metastatic niche, as well as the primary tumor microenvironment. Since the majority of patients with PDAC die from advanced metastatic disease (primarily in the liver), targeting tumor proliferation in this organ is of high clinical relevance. The precise deliver of the nanosystems to the liver in pre-clinical models of PDAC disrupted the metastatic niche, specifically converting pro-tumor macrophages in the liver into anti-tumor immune effector cells, effectively halting liver metastases and PDAC cell proliferation. Furthermore, the treatment showcased safety and biocompatibility, with no observed toxicity in vital organs, marking a crucial step forward in overcoming the challenges of PDAC treatment.
“Our findings open the door to a new era of cancer therapy. By harnessing the dual properties of these lipid nanosystems, we’re not just treating tumors—we’re reprogramming the immune landscape to fight cancer at its core” says Palencia-Campos. This promising approach could transform the treatment landscape for PDAC and other liver-metastatic cancers, offering hope to patients with metastatic disease.
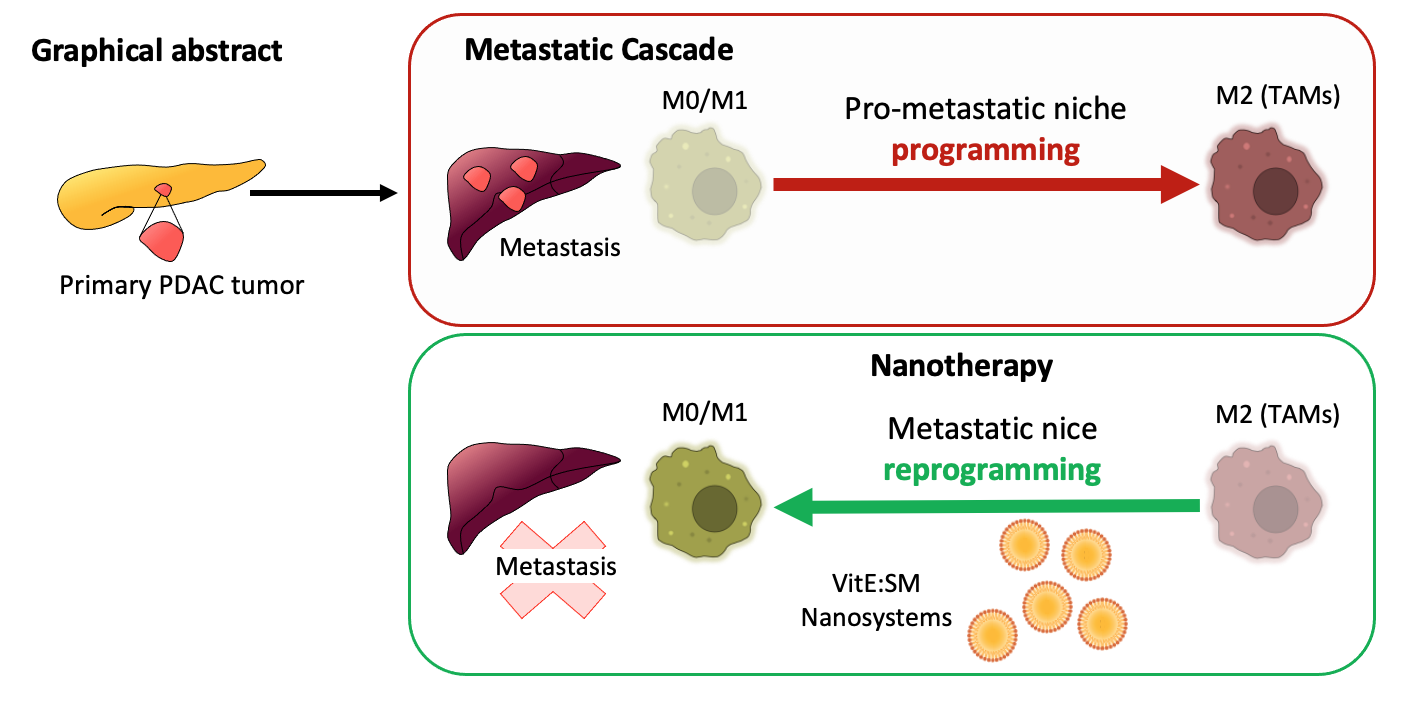
Figure. The PDAC metastatic cascade in the liver depends on the successful reprogramming of M0/M1 pro-inflammatory (anti-tumor) macrophages into M2-like tumor-associated macrophages (TAMs) within the pre-metastatic liver niche. The vitamin E and sphingomyelin (VitE:SM) nanosystems effectively accumulate in TAMs, and alone or with the TGF-βR1 inhibitor Galunisertib, can reprogram TAMs to their original M0/M1 pro-inflammatory and anti-tumor state, inhibiting PDAC cell metastatic seeding and proliferation.
This study was performed in collaboration with the team of Dr. María de la Fuente at the Instituto de Investigación Sanitaria de Santiago de Compostela (IDIS), and was funded by a EURONANOMED III - Joint Transnational Call for Proposals (2018)/PCI 2019-1 project entitled “Photoactivable nanoparticles to immunostimulate the tumour microenvironment in pancreatic cancer” (PANIPAC).
The paper: “Reprogramming tumor-associated macrophages with lipid nanosystems reduces PDAC tumor burden and liver metástasis” can be read HERE.