- New findings in the context of the immune synapse will serve to design therapeutic strategies aimed not only at autoimmune diseases but also at the processes of T-lymphocyte activation against cancer
The immune system comprised a vast diversity of cells, among which T cells play a fundamental role. T cell activation begins with the recognition by the T cell antigen receptor of antigens presented by antigen-presenting cells. This interaction triggers the formation of the immune synapse, characterized by the clustering of signaling molecules and adhesion receptors at the interface between both cells, which constitutes a platform leading to activation signals. Subsequently, the synapse architecture allows the focused secretion towards the antigen-presenting cell.
Actin filaments, which are essential for cell movement and shape changes, are also crucial in the formation and restructuring of the immune synapse. Formins are actin nucleators that regulate the lineal assembly of these filaments. These actin filaments are involved in processes such as lamellipodia formation and T cell spreading, facilitating effective communication with antigen-presenting cells. Formins promote the generation of linear actin filaments at the immune synapse, which contribute to the reorganization of the cytoskeleton during T cell activation. Several studies have revealed that specific formins, such as Dia1 and FMNL1, participate in the polarization of the secretory machinery towards the immune synapse. However, the molecular bases underlying polarized secretion and the role of FMNL1 and its regulators acting on this process are still unknown.
Ruiz-Navarro et al. show that the β isoform of FMNL1 is transiently recruited to the immune synapse following T cell antigen receptor activation in a process that is independent of S1086 residue phosphorylation. Once at the synapse, a PKCδ-mediated phosphorylation of FMNL1β at S1086 residue is crucial for FMNL1β control of two key processes: F-actin cortical reorganization resulting in hypodense actin areas in the central region of the synapse and the polarization of the secretory machinery towards the immune synapse. Acting in coordination, these two processes control the focused secretion of exosomes at the synapse.
In this publication: Formin-like 1 β phosphorylation at S1086 is necessary for secretory polarized traffic of exosomes at the immune synapse in Jurkat T lymphocytes , researchers from Idipaz, CNIO and ISCIII have also participated.
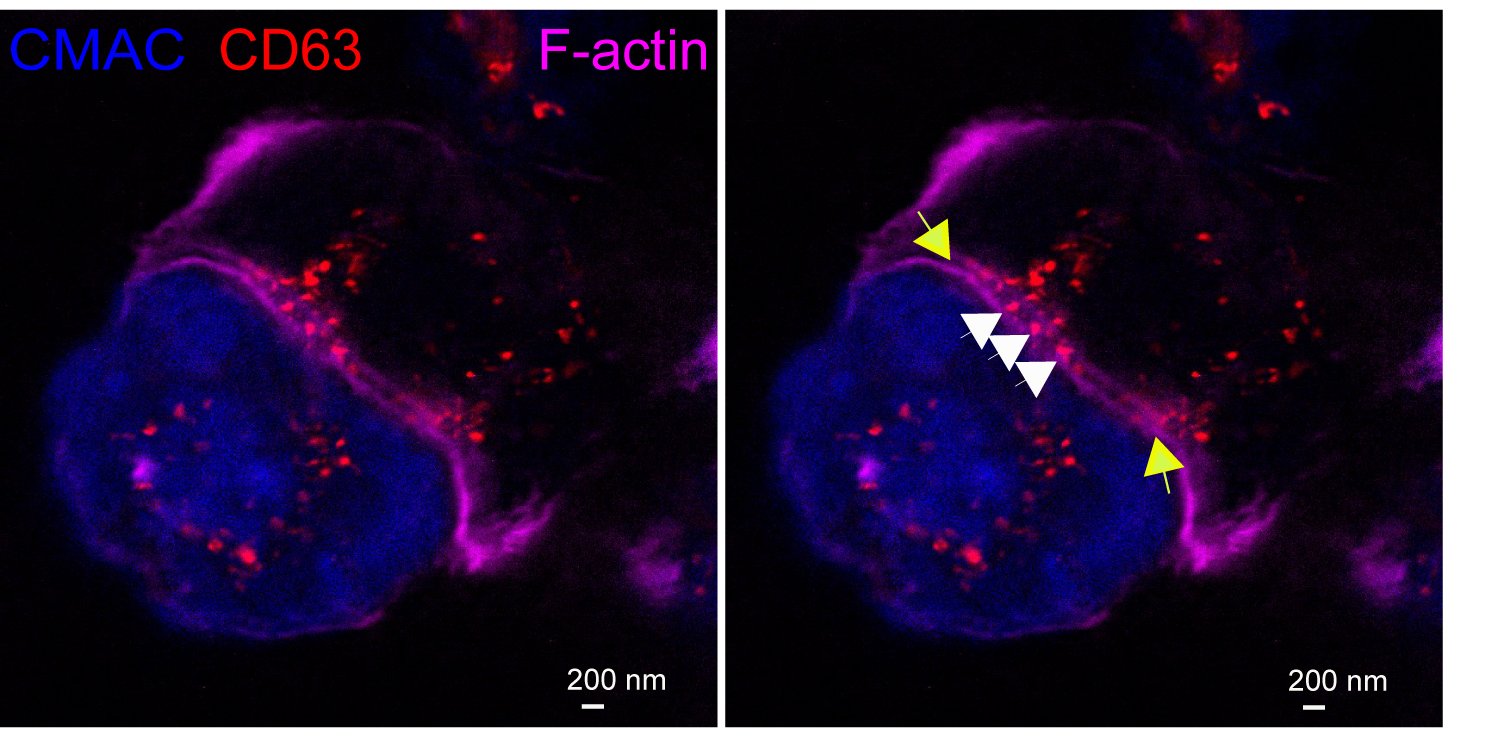
Exosomes at the Immune synapse.
A Jurkat T cell (top) forming an immune synapse with a Raji cell (bottom, blue). Multivesicular bodies within the Jurkat cell (red vesicles) are located nearby to the immune synapse. The yellow arrows label the edges of the synaptic cleft, which is the narrow, lane-shaped space between the two cells enclosed by the two F-actin-rich (magenta) plasma membranes. Some red nanovesicles (exosomes, white arrows) are located at the synaptic cleft. STED, super-resolution image.
Video: Immune synapse formation and polarized traffic of multivesicular bodies (MVB). GFP-CD63-expressing Jurkat C3 control clone cells were mixed with superantigen SEE-pulsed, CMAC-labeled Raji cells (blue) attached to slides to induce IS formation. Raji cells act as antigen-presenting cells, and the superantigen . The video (7 fps) shows an already established synapse (center) and an emerging one (left), along with the movement of GFP-CD63+ MVB in C3 cells towards the synaptic contact areas. CMAC (blue) and GFP-CD63 (green) merged channels are shown. The video is a representative example out of the 21 recorded synapses. Cell surface GFP-CD63 is observed, due to fusion of GFP-CD63+ MVB with the plasma membrane.