- Miguel Sánchez-Álvarez has contributed to a study published in Nature Communications, describing new mechanisms that enable adipocytes to expand safely for fat storage, preventing their rupture
- This discovery helps explain why certain pathologies are simultaneously associated with both excess fat and diseases characterized by impaired adipocyte expansion capacity, such as lipodystrophies, opening the door to potential treatments
Miguel Sánchez-Álvarez, head of the Cellular Compartmentalization, Homeostasis, and Inflammation group at the Instituto de Investigaciones Biomédicas Sols-Morreale (IIBM), CSIC-UAM, has contributed to a recent publication in Nature Communications with significant implications in the field of metabolism. This study describes an essential mechanism that allows adipocytes—cells responsible for fat storage in adipose tissue—to expand without rupturing, thereby protecting the body from the release of toxic molecules into surrounding tissues. This discovery paves the way for developing new therapeutic strategies for diseases related to the inability to store fat safely, including metabolic disorders associated with obesity.
Modern society is characterized by a lack of physical activity and excessive consumption of high-calorie foods. This energy surplus leads to overweight and obesity, increasing the risk of developing more serious conditions such as cardiovascular diseases, including atherosclerosis, and metabolic disorders like type 2 diabetes. Excess energy is stored in adipocytes as lipids (commonly known as fat), making these cells and adipose tissue crucial for metabolic health. Adipocytes can expand significantly to store excess energy as fat, preventing fat accumulation in organs such as the liver or the walls of blood vessels, particularly those of the heart and brain, where it could cause irreversible damage.
However, this process is not without risks: an excessive fat load can lead to adipocyte rupture, releasing toxic contents and triggering inflammation and metabolic disturbances. Interestingly, various syndromes characterized by limited adipose tissue size and/or function, such as lipodystrophies, often exhibit similar metabolic alterations. This highlights that proper adipocyte expansion is essential for their physical adaptation.
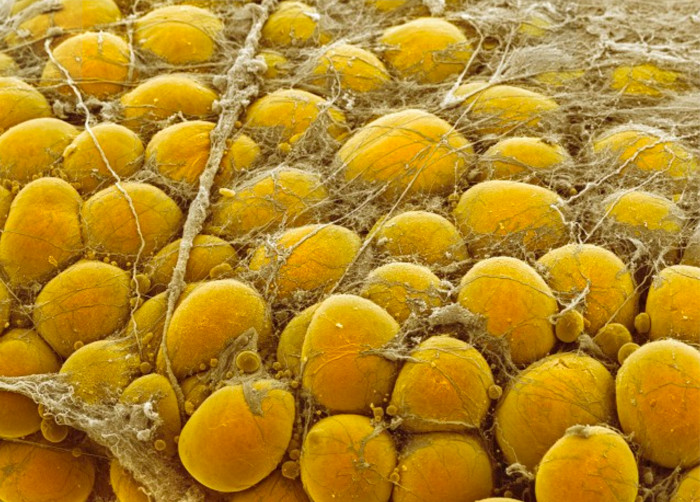
This study, coordinated by the Centro Nacional de Investigaciones Cardiovasculares (CNIC), focused on understanding how adipocytes physically adapt to withstand the mechanical stresses associated with their expansion, enabling the safe accumulation of fat. The researchers analyzed the role of caveolae, small invaginations in the cell membrane that act as sensors and buffers for mechanical tension. "When adipocytes accumulate fat and their surface is subjected to increased tension, caveolae flatten, releasing a 'membrane reservoir' that allows the cell to expand without rupturing. Conversely, when fat stores decrease, these structures regroup to reduce excess membrane and restore cellular stability," explain the authors of the study
Caveolae: More Than Just Structural Support
Caveolae not only protect adipocytes but also coordinate their metabolism, sending signals within the cell to adjust metabolic activity according to energy reserves. When these structures are absent or malfunctioning, adipocytes become stiffer, less efficient at storing energy, and more vulnerable to rupture. As a result, inflammation occurs, compromising the body's metabolic health.
A caveolae-associated protein known as Caveolin-1 (Cav1) plays a crucial role in caveolae reorganization, requiring phosphorylation to function properly. The researchers developed a transgenic mouse model expressing a modified form of Cav1 incapable of phosphorylation and discovered that its adipocytes lacked the ability to expand in response to excess fat, unlike those of normal mice.
These findings provide a deeper understanding of how adipocytes respond to mechanical forces associated with energy surplus. In individuals with obesity, this protective mechanism is essential for minimizing damage to the body.
This study, with Dr. María Aboy-Pardal as the first author, was led by Professor Miguel Ángel del Pozo from CNIC, with contributions from other CSIC institutions, including IIBM, the Instituto de Ciencias Materiales de Madrid (ICMM), the Centro de Investigaciones Biológicas Margarita Salas (CIB), also the Centro de Investigación en Medicina Molecular y Enfermedades Crónicas (CIMUS) and the Centro Nacional de Investigaciones Oncológicas (CNIO).

Aboy-Pardal MCM, Guadamillas MC, Guerrero CR, Català-Montoro M, Toledano-Donado M, Terrés-Domínguez S, Pavón DM, Jiménez-Jiménez V, Jiménez-Carretero D, Zamai M, Folgueira C, Cerezo A, Lolo FN, Nogueiras R, Sabio G, Sánchez-Álvarez M, Echarri A, García R, Del Pozo MA. Plasma membrane remodeling determines adipocyte expansion and mechanical adaptability. Nat Commun. Acepeted Nov 5 2024. In press. doi: 10.1038/s41467-024-54224-y
The paper can be found HERE