- A team led by Dr. Margarita Díaz-Guerra at IIBM identifies a new mechanism involved in ischemic stroke and develops an innovative therapy successfully tested in preclinical models
The research group Diagnostic and Neuroprotective Tools for Excitotoxicity and Brain Ischemia, led by Dr. Margarita Díaz-Guerra at the Instituto de Investigaciones Biomédicas Sols-Morreale (CSIC-UAM), has published a key finding in the journal Theranostics that sheds new light on ischemic stroke. The study reveals a previously unknown mechanism that regulates neuronal damage and the activation of glial cells—such as astrocytes and microglia—opening the door to a new therapeutic strategy that has already proven effective in preclinical models of this type of brain injury.
Stroke has major social and economic impacts. It is one of the leading causes of death, the primary cause of adult disability, and the second most common cause of dementia. Ischemic stroke, the most frequent type, results from an interruption in blood flow to the brain. Although treatments like thrombolysis and mechanical thrombectomy have brought significant advances, they are only applicable to a minority of patients. That’s why current efforts are focused on protecting the so-called ischemic penumbra—the brain tissue surrounding the infarct core that may still be salvaged.
“If no action is taken quickly, neurons in the penumbra can die as a result of a process called excitotoxicity, which disrupts the neurotrophic support essential for neuronal survival,” explains Dr. Díaz-Guerra. Another major contributor to ischemic brain damage is inflammation, which begins with the activation of resident immune cells in the brain, such as astrocytes and microglia.
In previous work, the group had already shown that the truncated neurotrophin receptor TrkB-T1—found mainly in neurons and astrocytes—plays a key role in both excitotoxicity and ischemic injury. Interestingly, 11 of the 23 amino acids that make up the intracellular region of the TrkB-T1 receptor form a sequence that is unique to this variant and highly conserved across species. “We hypothesized that this sequence could mediate interactions with other proteins that contribute to neurotoxicity and neuroinflammation. If so, it could represent a promising therapeutic target for stroke,” explains Lola Ugalde-Triviño, first author of the study and a PhD student at IIBM.
To test this hypothesis, the team designed special compounds called cell-penetrating peptides, which included the specific TrkB-T1 sequence. These peptides were able to cross the blood-brain barrier—a major obstacle for brain drug delivery—and reached both neurons and astrocytes. When used in primary cultures of these cell types, the peptides allowed the researchers to isolate and identify interacting proteins, revealing for the first time a TrkB-T1-specific interactome under both normal and excitotoxic conditions.
But the most striking results came later: treatment with these peptides not only reduced glial cell activation but also prevented neuronal death caused by excitotoxicity. This effect was observed both in primary brain cell cultures and in preclinical stroke models. In the latter, peptide treatment led to a significant reduction in infarct volume in both male and female mice. In males, this reduction was also associated with improved neurological performance in tests measuring motor coordination and balance. In females, however, no such correlation could be established, as they exhibited good neurological performance even with similar infarct volumes—suggesting potential sex differences in response
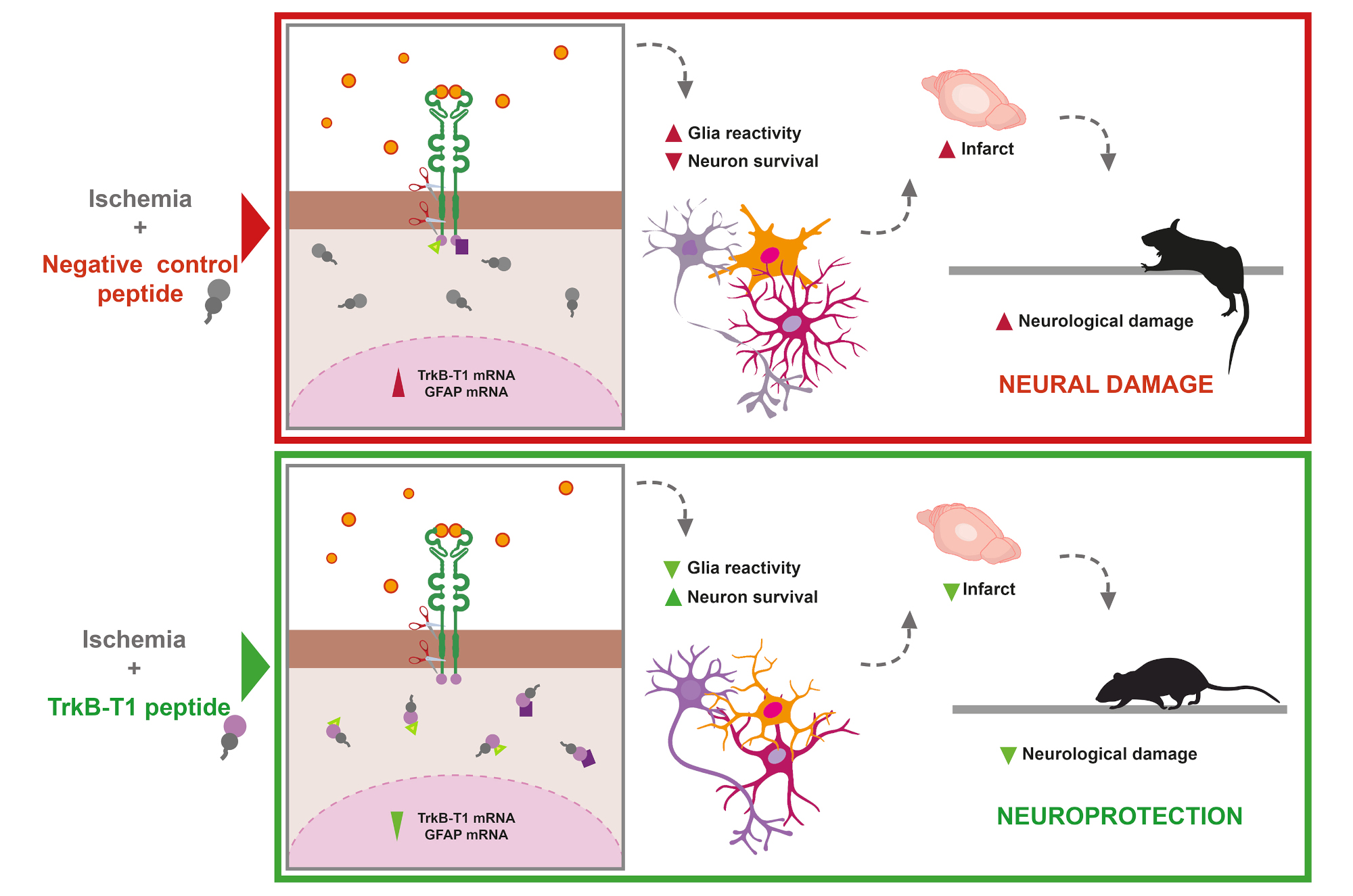
The image shows how the protective peptides designed from specific sequences of the TrkB-T1 receptor act, according to the model proposed by the research team.
Beyond stroke, the peptide developed in this study could have broader therapeutic applications, since excitotoxicity is a common feature of both acute injuries (such as stroke and traumatic spinal or brain injuries) and chronic neurodegenerative diseases. It also plays a role in disorders affecting hearing and vision.
In addition to Lola Ugalde-Triviño, the research team included Dr. Gonzalo S. Tejeda from the la School of Molecular Biosciences, University of Glasgow, and Dr. Gema M. Esteban-Ortega, now at the Centro de Biología Molecular Severo Ochoa (CBMSO). The study was funded by the Spanish State Research Agency (PID2019-105784RB-100 and PID2022-137710OB-I00).
Reference:
Lola Ugalde-Triviño, Gonzalo S. Tejeda†, Gema M. Esteban-Ortega†, and Margarita Díaz-Guerra. A brain-accessible peptide modulates stroke inflammatory response and neurotoxicity by targeting BDNF-receptor TrkB-T1 specific interactome. Theranostics, 2025. https://www.thno.org/v15p4654.htm
Cover image: Mouse brain after 24 hours of permanent focal ischemia. Reactive GFAP+ astrocytes (magenta) are shown forming the so-called glial scar in regions adjacent to the infarct core.